Acta Chimica Sinica ›› 2023, Vol. 81 ›› Issue (1): 42-63.DOI: 10.6023/A22100419 Previous Articles Next Articles
Review
投稿日期:2022-10-10
发布日期:2022-11-21
基金资助:
Kongxi Qiu, Jie Li, Haowen Ma, Wei Zhou( ), Qian Cai(
), Qian Cai( )
)
Received:2022-10-10
Published:2022-11-21
Contact:
*E-mail: Supported by:Share
Kongxi Qiu, Jie Li, Haowen Ma, Wei Zhou, Qian Cai. Recent Advances in the Construction of Nitrogen-Containing Heterocycles via Trapping Organocopper(I) Intermediates[J]. Acta Chimica Sinica, 2023, 81(1): 42-63.

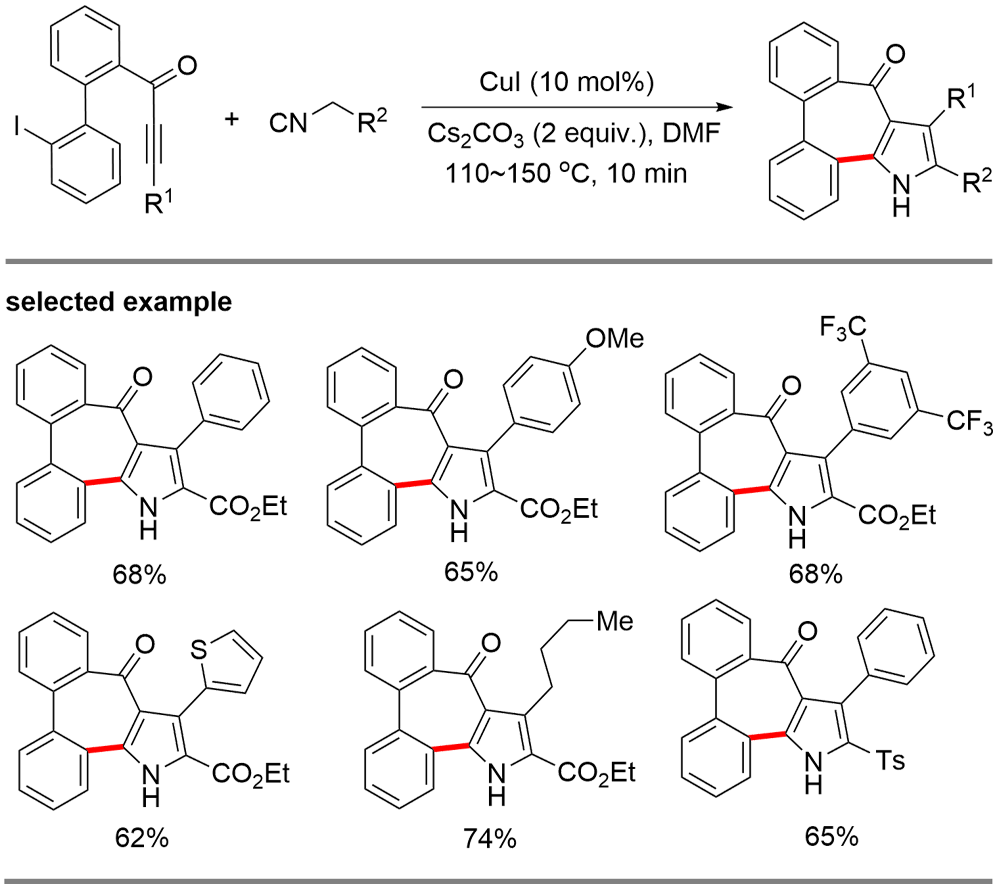
| [1] |
Gilman, H.; Jones, R. G.; Woods, L. A. J. Org. Chem. 1952, 17, 1630.
doi: 10.1021/jo50012a009 |
| [2] |
(a) Krause, N. Modern Organocopper Chemistry, Wiley-VCH, Weinheim, Germany, 2002.
pmid: 22111574 |
|
(b) Woodward, S. Chem. Soc. Rev. 2000, 29, 393.
doi: 10.1039/b002690p pmid: 22111574 |
|
|
(c) Yoshikai, N.; Nakamura, E. Chem. Rev. 2012, 112, 2339.
doi: 10.1021/cr200241f pmid: 22111574 |
|
|
(d) Tang, H.; Huang, D.; Li, Y.; Du, X.; Lian, H.; Chen, J. Acta Chim. Sinica 1994, 52, 306. (in Chinese)
pmid: 22111574 |
|
|
( 唐洪春, 黄道孝, 李玉林, 杜秀宝, 连洪寿, 陈冀胜, 化学学报 1994, 52, 306.)
pmid: 22111574 |
|
| [3] |
Kharasch, M. S.; Tawney, P. O. J. Am. Chem. Soc. 1941, 63, 2308.
doi: 10.1021/ja01854a005 |
| [4] |
For selected reviews, see: (a) Reymond, S.; Cossy, J. Chem. Rev. 2008, 108, 5359.
doi: 10.1021/cr078346g pmid: 25961125 |
|
(b) Stanley, L. M.; Sibi, M. P. Chem. Rev. 2008, 108, 2887.
doi: 10.1021/cr078371m pmid: 25961125 |
|
|
(c) Hein, J. E.; Fokin, V. V. Chem. Soc. Rev. 2010, 39, 1302.
doi: 10.1039/b904091a pmid: 25961125 |
|
|
(d) Khangaro, R. K.; Kaliappan, K. P. Eur. J. Org. Chem. 2013, 7664.
pmid: 25961125 |
|
|
(e) Hashimoto, T.; Maruoka, K. Chem. Rev. 2015, 115, 5366.
doi: 10.1021/cr5007182 pmid: 25961125 |
|
|
(f) Haldón, E.; Nicasio, M. C.; Pérez, P. J. Org. Biomol. Chem. 2015, 13, 9528.
doi: 10.1039/C5OB01457C pmid: 25961125 |
|
| [5] |
(a) Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057.
doi: 10.1021/jo011148j |
|
(b) Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596.
doi: 10.1002/1521-3773(20020715)41:14【-逻*辑*与-】#x00026;lt;2596::AID-ANIE2596【-逻*辑*与-】#x00026;gt;3.0.CO;2-4 |
|
|
(c) Dong, J.; Xu, L. Chin. J. Chem. 2020, 38, 414.
doi: 10.1002/cjoc.201900421 |
|
| [6] |
(a) Huisgen, R. Angew. Chem., Int. Ed. 1963, 2, 565.
|
|
(b) Huisgen, R. Angew. Chem., Int. Ed. 1963, 2, 633.
|
|
|
(c) Huisgen, R. Pure Appl. Chem. 1989, 61, 613.
doi: 10.1351/pac198961040613 |
|
| [7] |
For selected reviews, see: (a) Meldal, M.; Tornøe, C. W. Chem. Rev. 2008, 108, 2952.
doi: 10.1021/cr0783479 pmid: 26796328 |
|
(b) Tiwari, V. K.; Mishra, B. B.; Mishra, K. B.; Mishra, N.; Singh, A. S.; Chen, X. Chem. Rev. 2016, 116, 3086.
doi: 10.1021/acs.chemrev.5b00408 pmid: 26796328 |
|
|
(c) Wang, X.; Huang, B.; Liu, X.; Zhan, P. Drug Discov. Today 2016, 21, 118.
doi: 10.1016/j.drudis.2015.08.004 pmid: 26796328 |
|
|
(d) Döhler, D.; Michael, P.; Binder, W. H. Acc. Chem. Res. 2017, 50, 2610.
doi: 10.1021/acs.accounts.7b00371 pmid: 26796328 |
|
|
(e) Meldal, M.; Diness, F. Trends Chem. 2020, 2, 569.
doi: 10.1016/j.trechm.2020.03.007 pmid: 26796328 |
|
| [8] |
Nolte, C.; Mayer, P.; Straub, B. F. Angew. Chem., Int. Ed. 2007, 46, 2101.
doi: 10.1002/anie.200604444 |
| [9] |
Wu, Y.-M.; Deng, J.; Li, Y.; Chen, Q.-Y. Synthesis 2005, 8, 1314.
|
| [10] |
Cassidy, M. P.; Raushel, J.; Fokin, V. V. Angew. Chem., Int. Ed. 2006, 45, 3154.
doi: 10.1002/anie.200503805 |
| [11] |
Zhang, X.; Hsung, R. P.; Li, H. Chem. Commun. 2007, 2420.
|
| [12] |
Li, L.; Zhang, G.; Zhu, A.; Zhang, L. J. Org. Chem. 2008, 73, 3630.
doi: 10.1021/jo800035v |
| [13] |
Li, L.; Li, R.; Zhu, A.; Zhang, G.; Zhang, L. Synlett 2011, 874.
|
| [14] |
Malnuit, V.; Duca, M.; Manout, A.; Bougrin, K.; Benhida, R. Synlett 2009, 2123.
|
| [15] |
(a) Wang, B.; Ahmed, M. N.; Zhang, J.; Chen, W.; Wang, X.; Hu, Y. Tetrahedron Lett. 2013, 54, 6097.
doi: 10.1016/j.tetlet.2013.08.121 pmid: 24032422 |
|
(b) Wang, B.; Zhang, J.; Wang, X.; Liu, N.; Chen, W.; Hu, Y. J. Org. Chem. 2013, 78, 10519.
doi: 10.1021/jo401629x pmid: 24032422 |
|
|
(c) Wang, B.; Liu, N.; Shao, C.; Zhang, Q.; Wang, X.; Hu, Y. Adv. Synth. Catal. 2013, 355, 2564.
doi: 10.1002/adsc.201300307 pmid: 24032422 |
|
|
(d) Wang, B.; Liu, N.; Chen, W.; Huang, D.; Wang, X.; Hu, Y. Adv. Synth. Catal. 2015, 357, 401.
doi: 10.1002/adsc.201400471 pmid: 24032422 |
|
| [16] |
Yan, R.; Sander, K.; Galante, E.; Rajkumar, V.; Badar, A.; Robson, M.; El-Emir, E.; Lythgoe, M. F.; Pedley, R. B.; Årstad, E. J. Am. Chem. Soc. 2013, 135, 703.
doi: 10.1021/ja307926g |
| [17] |
Chen, Z.; Zhu, J.; Xie, H.; Li, S.; Wu, Y.; Gong, Y. Adv. Synth. Catal. 2010, 352, 1296.
doi: 10.1002/adsc.200900875 |
| [18] |
Cai, Q.; Zhou, W. Chin. J. Chem. 2020, 38, 879.
doi: 10.1002/cjoc.202000075 |
| [19] |
Yan, J.; Zhou, F.; Qin, D.; Cai, T.; Ding, K.; Cai, Q. Org. Lett. 2012, 14, 1262.
doi: 10.1021/ol300114w |
| [20] |
Hooyberghs, G.; De Coster, H.; Vachhani, D. D.; Ermolat’ev, D. S.; Van der Eycken, E. V. Tetrahedron 2013, 69, 4331.
doi: 10.1016/j.tet.2013.03.031 |
| [21] |
Vachhani, D. D.; Kumar, A.; Modha, S. G.; Sharma, S. K.; Parmar, V. S.; Van der Eycken, E. V. Eur. J. Org. Chem. 2013, 1223.
|
| [22] |
Reddy, A. S.; Reddy, M. N.; Swamy, K. C. K. RSC Adv. 2014, 4, 28359.
doi: 10.1039/C4RA03503H |
| [23] |
An, Y.; He, H.; Liu, T.; Zhang, Y.; Lu, X.; Cai, Q. Synthesis 2017, 49, 3863.
doi: 10.1055/s-0036-1590791 |
| [24] |
Xiao, G.; Wu, K.; Zhou, W.; Cai, Q. Adv. Synth. Catal. 2021, 363, 4988.
doi: 10.1002/adsc.202100955 |
| [25] |
Cai, Q.; Yan, J.; Ding, K. Org. Lett. 2012, 14, 3332.
doi: 10.1021/ol301307x |
| [26] |
Ouyang, Y.; Si, H.; Zhu, C.; Zhong, L.; Ma, H.; Li, Z.; Xiong, H.; Liu, T.; Liu, Z.; Zhang, Z.; Zhang, Z.; Cai, Q. J. Med. Chem. 2022, 65, 7833.
doi: 10.1021/acs.jmedchem.2c00271 |
| [27] |
Bag, S. S.; Das, S. K.; Gogoi, H. Tetrahedron, 2018, 74, 2218.
doi: 10.1016/j.tet.2018.03.037 |
| [28] |
Reddy, M. N.; Swamy, K. C. K. Eur. J. Org. Chem. 2012, 2013.
|
| [29] |
Wei, F.; Wang, W.; Ma, Y.; Tung, C.-H.; Xu, Z. Chem. Commun. 2016, 52, 14188.
doi: 10.1039/C6CC06194J |
| [30] |
Ackermann, L.; Potukuchi, H. K.; Landsberg, D.; Vicente, R. Org. Lett. 2008, 10, 3081.
doi: 10.1021/ol801078r pmid: 18549230 |
| [31] |
Qian, W.; Wang, H.; Allen, J. Angew. Chem., Int. Ed. 2013, 52, 10992.
doi: 10.1002/anie.201305970 |
| [32] |
Pericherla, K.; Jha, A.; Khungar, B.; Kumar, A. Org. Lett. 2013, 15, 4304.
doi: 10.1021/ol401655r |
| [33] |
Liu, Z.; Zhu, D.; Luo, B.; Zhang, N.; Liu, Q.; Hu, Y.; Pi, R.; Huang, P.; Wen, S. Org. Lett. 2014, 16, 5600.
doi: 10.1021/ol502654a |
| [34] |
Lautens, M.; Larin, E. M. Angew. Chem., Int. Ed. 2019, 58, 13438.
doi: 10.1002/anie.201907448 |
| [35] |
Wei, F.; Li, H.; Song, C.; Ma, Y.; Zhou, L.; Tung, C.-H.; Xu, Z. Org. Lett. 2015, 17, 2860.
doi: 10.1021/acs.orglett.5b01342 |
| [36] |
(a) Zhang, Z.; Zhou, Q.; Ye, F.; Xia, Y.; Wu, G.; Hossain, M. L.; Zhang, Y.; Wang, J. Adv. Synth. Catal. 2015, 357, 2277.
doi: 10.1002/adsc.201500377 |
|
(b) Zhang, Z.; Zhou, Q.; Yu, W.; Li, T.; Zhang, Y.; Wang, J. Chin. J. Chem. 2017, 35, 387.
doi: 10.1002/cjoc.201600888 |
|
| [37] |
Chen, F.-J.; Mamidipalli, P.; Sabbasani, V. R.; Liu, H.; Xia, Y.; Lee, D. Org. Chem. Front. 2021, 8, 6095.
doi: 10.1039/D1QO01112J |
| [38] |
Wang, W.; Wei, F.; Ma, Y.; Tung, C.-H.; Xu, Z. Org. Lett. 2016, 18, 4158.
doi: 10.1021/acs.orglett.6b02199 |
| [39] |
(a) Zhou, W.; Zhang, M.; Li, H.; Chen, W. Org. Lett. 2017, 19, 10.
doi: 10.1021/acs.orglett.6b02850 pmid: 27966996 |
|
(b) Wu, F.; Zhou, W.; Chen, K.; Chen, W.; Liu, M.; Wu, H. Adv. Synth. Catal. 2018, 360, 2435.
doi: 10.1002/adsc.201800394 pmid: 27966996 |
|
| [40] |
Cheung, K. P. S.; Tsui, G. C. Org. Lett. 2017, 19, 2881.
doi: 10.1021/acs.orglett.7b01116 |
| [41] |
Wu, W.; Wang, J.; Wang, Y.; Huang, Y.; Tan, Y.; Weng, Z. Angew. Chem., Int. Ed. 2017, 56, 10476.
doi: 10.1002/anie.201705620 |
| [42] |
Wang, W.; Peng, X.; Wei, F.; Tung, C.-H.; Xu, Z. Angew. Chem., Int. Ed. 2016, 55, 649.
doi: 10.1002/anie.201509124 |
| [43] |
Wang, W.; Huang, S.; Yan, S.; Sun, X.; Tung, C.-H.; Xu, Z. Chin. J. Chem. 2020, 38, 445.
doi: 10.1002/cjoc.201900556 |
| [44] |
Reddy, R. J.; Waheed, M.; Kumari, A. H.; Krishna, G. R. Adv. Synth. Catal. 2022, 364, 319.
doi: 10.1002/adsc.202101256 |
| [45] |
Wei, F.; Zhou, T.; Ma, Y.; Tung, C.-H.; Xu, Z. Org. Lett. 2017, 19, 2098.
doi: 10.1021/acs.orglett.7b00701 |
| [46] |
Yang, D.; Fu, N.; Liu, Z.; Li, Y.; Chen, B. Synlett 2007, 278.
|
| [47] |
Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. Synlett 2012, 23, 2179.
doi: 10.1055/s-0031-1290445 |
| [48] |
Li, L.; Hao, G.; Zhu, A.; Fan, X.; Zhang, G.; Zhang, L. Chem. Eur. J. 2013, 19, 14403.
doi: 10.1002/chem.201303324 |
| [49] |
(a) Li, L.; Fan, X.; Zhang, Y.; Zhu, A.; Zhang, G. Tetrahedron 2013, 69, 9939.
doi: 10.1016/j.tet.2013.09.093 |
|
(b) Li, L.-J.; Zhang, Y.-Q.; Zhang, Y.; Zhu, A.-L.; Zhang, G.-S. Chin. Chem. Lett. 2014, 25, 1161.
doi: 10.1016/j.cclet.2014.03.004 |
|
| [50] |
Selvaraju, M.; Sun, C.-M. Adv. Synth. Catal. 2014, 356, 1329.
doi: 10.1002/adsc.201301013 |
| [51] |
(a) Peringer, F.; do Nascimento, J. E. R.; Abib, P. B.; Barcellos, T.; Van der Eycken, E. V.; Perin, G.; Jacob, R. G. Alves, D. Eur. J. Org. Chem. 2017, 2579.
|
|
(b) Aquino, T. B.; do Nascimento, J. E. R.; Dias, Í. F. C.; de Oliveira, D. H.; Barcellos, T.; Lenardão, E. J.; Perin, G.; Alves, D.; Jacob, R. J. Tetrahedron Lett. 2018, 59, 1080.
doi: 10.1016/j.tetlet.2018.01.072 |
|
| [52] |
Xu, J.; Song, Q. Org. Chem. Front. 2017, 4, 938.
doi: 10.1039/C6QO00725B |
| [53] |
Yu, X.; Xu, J.; Zhou, Y.; Song, Q. Org. Chem. Front. 2018, 5, 2463.
doi: 10.1039/C8QO00590G |
| [54] |
Wang, X.-X.; Xin, Y.; Li, Y.; Xia, W.-J.; Zhou, B.; Ye, R.-R.; Li, Y.-M. J. Org. Chem. 2020, 85, 3576
doi: 10.1021/acs.joc.9b03285 |
| [55] |
(a) Navarro, Y.; López, J. G.; Iglesias, M. J.; Ortiz, F. L. Org. Lett. 2021, 23, 334.
doi: 10.1021/acs.orglett.0c03838 |
|
(b) Navarro, Y.; Jiménez, I. H.; Iglesias, M. J.; Ortiz, F. L. Synthesis 2022, 54, 199.
doi: 10.1055/a-1577-5999 |
|
| [56] |
(a) Kamijo, S.; Kanazawa, C.; Yamamoto, Y. J. Am. Chem. Soc. 2005, 127, 9260.
pmid: 15969607 |
|
(b) Larionov, O. V.; de Meijere, A. Angew. Chem., Int. Ed. 2005, 44, 5664.
doi: 10.1002/anie.200502140 pmid: 15969607 |
|
| [57] |
Cai, Q.; Zhou, F.; Xu, T.; Fu, L.; Ding, K. Org. Lett. 2011, 13, 340.
doi: 10.1021/ol102826f |
| [58] |
Zhou, F.; Fu, L.; Wei, J.; Ding, K.; Cai, Q. Synthesis 2011, 3037.
|
| [59] |
Zhou, F.; Liu, J. Ding, K.; Liu, J.; Cai, Q. J. Org. Chem. 2011, 76, 5346.
doi: 10.1021/jo2006939 |
| [60] |
Zheng, D.; Liu, T.; Liu, X.; Fan, X.; Wu, J. J. Org. Chem. 2016, 81, 9428.
doi: 10.1021/acs.joc.6b01669 |
| [61] |
Ouyang, Y.; Wu, K.; Zhou, W.; Cai, Q. Org. Chem. Front. 2021, 8, 2456.
doi: 10.1039/D0QO01657H |
| [62] |
Kinugasa, M.; Hashimoto, S. J. Chem. Soc. Chem. Commun. 1972, 466.
|
| [63] |
(a) Santoro, S.; Himo, F. J. Org. Chem. 2021, 86, 10665.
doi: 10.1021/acs.joc.1c01351 |
|
(b) Malig, T. C.; Yu, D.; Hein, J. E. J. Am. Chem. Soc. 2018, 140, 9167.
doi: 10.1021/jacs.8b04635 |
|
|
(c) Santoro, S.; Liao, R.-Z.; Marcelli, T.; Hammar, P.; Himo, F. J. Org. Chem. 2015, 80, 2649.
doi: 10.1021/jo502838p |
|
| [64] |
Shintani, R.; Fu, G. C. Angew. Chem., Int. Ed. 2003, 42, 4082.
doi: 10.1002/anie.200352103 |
| [65] |
Shu, T.; Zhao, L.; Li, S.; Chen, X.-Y.; von Essen, C.; Rissanen, K.; Enders, D. Angew. Chem., Int. Ed. 2018, 57, 10985.
doi: 10.1002/anie.201806931 |
| [66] |
Qi, J.; Wei, F.; Huang, S.; Tung, C.-H.; Xu, Z. Angew. Chem., Int. Ed. 2021, 60, 4561.
doi: 10.1002/anie.202013450 |
| [67] |
(a) Qi, J.; Wei, F.; Tung, C.-H.; Xu, Z. Angew. Chem., Int. Ed. 2021, 60, 13814.
doi: 10.1002/anie.202100601 |
|
(b) Qi, J.; Tung, C.-H.; Xu, Z. Trends Chem. 2021, 3, 984.
doi: 10.1016/j.trechm.2021.08.004 |
|
| [68] |
Zhong, X.; Huang, M.; Xiong, H.; Liang, Y.; Zhou, W.; Cai, Q. Angew. Chem., Int. Ed. 2022, DOI: 10.1002/anie.202208323.
doi: 10.1002/anie.202208323 |
| [1] | Zhanglong Yu, Zhongliang Li, Changjiang Yang, Qiangshuai Gu, Xinyuan Liu. Research Progress on Copper-Catalyzed Enantioselective Desymmetrization of Diols★ [J]. Acta Chimica Sinica, 2023, 81(8): 955-966. |
| [2] | Qinghao Xu, Lipu Wei, Zhen Zhang, Bin Xiao. Copper Promoted Synthesis of Tetraalkylgermanes from Germanium Electrophiles and Alkyl Bromides※ [J]. Acta Chimica Sinica, 2022, 80(4): 428-431. |
| [3] | Da Yang, Longli Zhang, Huan Liu, Chaohe Yang. Co-catalysis over Bi-functional Ligand Based Ir-catalyst for Tandem Hydroformylation-acetalization Reaction [J]. Acta Chimica Sinica, 2021, 79(5): 658-662. |
| [4] | Zhang Ronghua, Xu Bing, Zhang Zhanming, Zhang Junliang. Ming-Phos/Copper(I)-Catalyzed Asymmetric[3+2] Cycloaddition of Azomethine Ylides with Nitroalkenes [J]. Acta Chimica Sinica, 2020, 78(3): 245-249. |
| [5] | Liang Huan, Gou Along, Gao Zhupeng, Lei Linsheng, Wang Bowen, Yu Lan, Xu Xuetao, Wang Shaohua. A New Strategy for the Synthesis of Tertiary Amides via a Copper-Catalyzed Decyanation Reaction of N,N-Disubstituted 2-Aminomalononitriles [J]. Acta Chimica Sinica, 2020, 78(10): 1064-1068. |
| [6] | Lin, Fengguirong, Liang, Yujie, Li, Xinyao, Song, Song, Jiao, Ning. Copper-catalyzed ortho C-H Azidation of Anilines Using Molecular Oxygen as Terminal Oxidant [J]. Acta Chimica Sinica, 2019, 77(9): 906-910. |
| [7] | Cheng, Zhongming, Chen, Pinhong, Liu, Guosheng. Enantioselective Cyanation of Remote C—H Bonds via Cooperative Photoredox and Copper Catalysis [J]. Acta Chimica Sinica, 2019, 77(9): 856-860. |
| [8] | Zhang, Heng, Mou, Xueqing, Chen, Gong, He, Gang. Copper-catalyzed Intramolecular Aminoperfluoroalkylation Reaction of O-Homoallyl Benzimidates [J]. Acta Chimica Sinica, 2019, 77(9): 884-888. |
| [9] | Huang Pei-Qiang. Direct Transformations of Amides: Tactics and Recent Progress [J]. Acta Chim. Sinica, 2018, 76(5): 357-365. |
| [10] | Ren Zhiwen, Ren Nan, Zhang Faguang, Ma Junan. Facile Synthesis of Fluorinated Isoxazoles via Consecutive Double C—F Bond Cleavage [J]. Acta Chim. Sinica, 2018, 76(12): 940-944. |
| [11] | Li Xue-Fei, Lin Jin-Shun, Wang Jian, Li Zhong-Liang, Gu Qiang-Shuai, Liu Xin-Yuan. Cu/Chiral Phosphoric Acid-Catalyzed Asymmetric Radical-Initiated Aminoarylation of Alkenes [J]. Acta Chimica Sinica, 2018, 76(11): 878-882. |
| [12] | Li Xin-Ling, Wang Jia-Qi, Li Long, Yin Ying-Wu, Ye Long-Wu. Facile Synthesis of 2H-Pyrroles: Combination of Gold Catalysis and Lewis Acid Catalysis [J]. Acta Chim. Sinica, 2016, 74(1): 49-53. |
| [13] | Wang Xuebin, Wang Xiaoli, Hu Jing, Wang Zhaoya, Pimpalpalle Tukaram M, Linker Torsten, Yin Jian. Study on the Synthesis of Novel Sugar Amino Acids [J]. Acta Chim. Sinica, 2015, 73(7): 699-704. |
| [14] | Jian-Feng Zheng, Zhi-Qiang Xie, Xin-Jian Chen, Pei-Qiang Huang. Direct Transformation of Amides: Reductive Cycloaddition of Secondary Amides with Danishefsky Diene [J]. Acta Chim. Sinica, 2015, 73(7): 705-715. |
| [15] | Hu Qi, Li Yuxiang, Wang Jingyuan, Li Yapeng. Synthetic of pH-Sensitive PEG-b-PHEMA(His) Polymers andthe Study of Micelle [J]. Acta Chim. Sinica, 2015, 73(5): 416-422. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||