Abstract
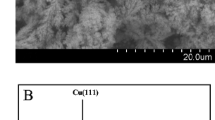
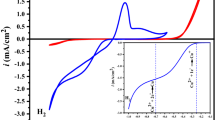
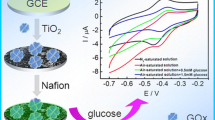
The wetting properties of an electrode surface are of significant importance to the performance of electrochemical devices because electron transfer occurs at the electrode/electrolyte interface. Described in this paper is a low-cost metal oxide electrocatalyst (CuO)-based high-performance sensing device using an enzyme electrode with a solid/liquid/air triphase interface in which the oxygen level is constant and sufficiently high. We apply the sensing device to detect glucose, a model test analyte, and demonstrate a linear dynamic range up to 50 mM, which is about 25 times higher than that obtained using a traditional enzyme electrode with a solid/liquid diphase interface. Moreover, we show that sensing devices based on a triphase assaying interface are insensitive to the significant oxygen level fluctuation in the analyte solution.

Similar content being viewed by others
References
Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8.
Cassie, A. B. D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551.
Feng, L.; Li, S. H.; Li, Y. S.; Li, H. J.; Zhang, L. J.; Zhai, J.; Song, Y. L.; Liu, B. Q.; Jiang, L.; Zhu, D. B. Superhydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860.
Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460.
Liu, T. Y.; Kim, C. J. Turning a surface superrepellent even to completely wetting liquids. Science 2014, 346, 1096–1100.
Stojanovic, A.; Artus, G. R. J.; Seeger, S. Micropatterning of superhydrophobic silicone nanofilaments by a near-ultraviolet Nd:YAG laser. Nano Res. 2010, 3, 889–894.
Li, J.; Hou, Y. M.; Liu, Y. H.; Hao, C. L.; Li, M. F.; Chaudhury, M. K.; Yao, S. H.; Wang, Z. K. Directional transport of high-temperature Janus droplets mediated by structural topography. Nat. Phys. 2016, 12, 606–612.
Lu, Y.; Sathasivam, S.; Song, J. L.; Crick, C. R.; Carmalt, C. J.; Parkin, I. P. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135.
Wang, S. T.; Liu, K. S.; Yao, X.; Jiang, L. Bioinspired surfaces with superwettability: New insight on theory, design, and applications. Chem. Rev. 2015, 115, 8230–8293.
Gwon, H. J.; Park, Y.; Moon, C. W.; Nahm, S.; Yoon, S.-J.; Kim, S. Y.; Jang, H. W. Superhydrophobic and antireflective nanograss-coated glass for high performance solar cells. Nano Res. 2014, 7, 670–678.
Su, B.; Wang, S. T.; Song, Y. L.; Jiang, L. A miniature droplet reactor built on nanoparticle-derived superhydrophobic pedestals. Nano Res. 2011, 4, 266–273.
Deng, X.; Mammen, L.; Butt, H. J.; Vollmer, D. Candle soot as a template for a transparent robust superamphiphobic coating. Science 2012, 335, 67–70.
Aebisher, D.; Bartusik, D.; Liu, Y.; Zhao, Y. Y.; Barahman, M.; Xu, Q. F.; Lyons, A. M.; Greer, A. Superhydrophobic photosensitizers. Mechanistic studies of 1O2 generation in the plastron and solid/liquid droplet interface. J. Am. Chem. Soc. 2013, 135, 18990–18998.
Lei, Y. J.; Sun, R. Z.; Zhang, X. C.; Feng, X. J.; Jiang, L. Oxygen-rich enzyme biosensor based on superhydrophobic electrode. Adv. Mater. 2016, 28, 1477–1481.
Lu, Z. Y.; Xu, W. W.; Ma, J.; Li, Y. J.; Sun, X. M.; Jiang, L. Superaerophilic carbon-nanotube-array electrode for highperformance oxygen reduction reaction. Adv. Mater. 2016, 28, 7155–7161.
Wang, S. S.; Wu, Y. C.; Kan, X. N.; Su, B.; Jiang, L. Regular metal sulfide microstructure arrays contributed by ambient-connected gas matrix trapped on superhydrophobic surface. Adv. Funct. Mater. 2014, 24, 7007–7013.
Guilbault, G. G.; Lubrano, G. J. An enzyme electrode for the amperometric determination of glucose. Anal. Chim. Acta 1973, 64, 439–455.
Wilson, G. S.; Hu, Y. B. Enzyme-based biosensors for in vivo measurements. Chem. Rev. 2000, 100, 2693–2704.
Heller, A.; Feldman, B. Electrochemical glucose sensors and their applications in diabetes management. Chem. Rev. 2008, 108, 2482–2505.
Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825.
Chu, Z. Y.; Shi, L.; Liu, Y.; Jin, W. Q.; Xu, N. P. In-situ growth of micro-cubic Prussian blue-TiO2 composite film as a highly sensitive H2O2 sensor by aerosol co-deposition approach. Biosens. Bioelectron. 2013, 47, 329–334.
Zhang, L.; Ni, Y. H.; Wang, X. H.; Zhao, G. C. Direct electrocatalytic oxidation of nitric oxide and reduction of hydrogen peroxide based on α-Fe2O3 nanoparticles-chitosan composite. Talanta 2010, 82, 196–201.
Asif, M. H.; Ali, S. M.; Nur, O.; Willander, M.; Brannmark, C.; Strålfors, P.; Englund, U. H.; Elinder, F.; Danielsson, B. Functionalised ZnO-nanorod-based selective electrochemical sensor for intracellular glucose. Biosens. Bioelectron. 2010, 25, 2205–2211.
Hahn, Y. B.; Ahmad, R.; Tripathy, N. Chemical and biological sensors based on metal oxide nanostructures. Chem. Commun. 2012, 48, 10369–10385.
Umar, A.; Rahman, M. M.; Al-Hajry, A.; Hahn, Y. B. Enzymatic glucose biosensor based on flower-shaped copper oxide nanostructures composed of thin nanosheets. Electrochem. Commun. 2009, 11, 278–281.
Li, C. L.; Kurniawan, M.; Sun, D. L.; Tabata, H.; Delaunay, J. J. Nanoporous CuO layer modified Cu electrode for high performance enzymatic and non-enzymatic glucose sensing. Nanotechnology 2015, 26, 015503.
Zhang, J.; Sheng, X.; Cheng, X. Q.; Chen, L. P.; Jin, J.; Feng, X. J. Robust electrochemical metal oxide deposition using an electrode with a superhydrophobic surface. Nanoscale 2017, 9, 87–90.
Cussler, E. L. Diffusion: Mass Transfer in Fluid Systems; Cambridge University Press: Cambridge, 1984.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 21371178), the Jiangsu Province Science Foundation for Distinguished Young Scholars (No. BK20150032), and the Chinese Thousand Youth Talents Program (No. YZBQF11001).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhang, J., Sheng, X., Jin, J. et al. High performance metal oxide based sensing device using an electrode with a solid/liquid/air triphase interface. Nano Res. 10, 2998–3004 (2017). https://doi.org/10.1007/s12274-017-1510-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1510-x